CONTACT

01
Katecho
Des Moine, IA
Katecho is an experienced medical device manufacturing company, based in Des Moines, IA. With a commitment to innovation and design, the team at Katecho offers comprehensive services for product design, process design, printing, manufacturing, and logistics. They are certified by ISO 13485:2003 as well as Japanese and Korean government authorities. The company also specializes in hydrogel and pharmaceutical manufacturing solutions. Their passion for building products with purpose has led them to successful products delivered with precision to customers around the world. From product development through the delivery of finished goods, Katecho stands out from other US-based contract medical device manufacturers, providing exceptional value to their clients.

02
Medical Murray
North Barrington, IL
Medical Murray is a contract development and manufacturing organization for minimally invasive medical devices and implants based in the US. They specialize in a range of technologies, from complex catheter delivery systems to steerable and deflectable balloon catheters, and sensor integration with implants. Their experienced team is dedicated to providing quality service, from concept through commercialization. Medical Murray can be trusted for its ISO 10555 Catheter Testing, ISO 25539 Stent Testing, ISO 80369 Luer Testing, and Power Injection testing capabilities, all of which comply with the highest safety standards. With their expertise and collaborative approach, they are sure to provide excellent products that will improve lives around the world.

03
MME Group
St. Paul, MN

MME Group is a full-service injection molder and contract manufacturer based in St. Paul, Minnesota. They specialize in providing comprehensive services to medical device manufacturers, from engineering through assembly. Their vertical integration advantage enables them to provide on-site tooling design and builds, custom plastic and silicone injection molding, secondary operations, high-level assembly, packaging, inventory management, supply chain management, and distribution – all under one roof. In addition to their excellent services, they are ISO 13485:2016 certified, ITAR registered, and have certifications for UL, CE, and TUV, making them the ideal choice for any of your medical device needs. Not only are they trusted by some of the biggest names in manufacturing, but they also offer superior quality control processes, which ensure that each product conforms to industry standards before being shipped out.

04
GCM
Union City, CA

GCM Precision Manufacturing is a trusted medical device manufacturer based in the United States. They specialize in providing high-quality and reliable components for medical technology, surgical robotics, aerospace, and industrial markets. Their services include engineering, supply chain management, precision machining, robotic welding and joining technologies, automated sheet metal fabrication, precision assembly, and more. With over 40 years of experience in key end markets such as MedTech, Aerospace, and Industrial applications, they continue to invest in their talent and technology infrastructure to provide industry-leading solutions. Their commitment to quality has been unwavering, as seen through their continual improvement of Quality Management Systems, designed to exceed customer expectations.

05
ViewRay
Oakwood Village, OH

ViewRay is a medical device manufacturer in the US, specializing in MRI-guided radiation therapy. Their groundbreaking work has enabled them to treat over 31,000 patients worldwide with their advanced A3i system. Patients benefit from improved imaging capabilities and increased accuracy in radiation delivery for various cancers such as pancreas, prostate, lung, liver, and breast cancer. Clinicians can also take advantage of the company's extensive clinical trials and Investigator-Initiated Research programs to further explore the possibilities offered by ViewRay's technology. The leadership team at ViewRay is dedicated to providing innovative solutions that make a real difference in cancer care.

06
NAGL Medtech
Miramar, FL
NAGL MedTech is a US-based medical device manufacturer specializing in transforming healthcare through cutting-edge technologies. The company provides comprehensive services for development, manufacturing, and consulting, offering clients the full spectrum of solutions necessary to create innovative medical devices. NAGL MedTech's team of experts is knowledgeable in every aspect of the industry and collaborate closely with their clients to provide strategic solutions tailored to their challenges and objectives. From neurovascular steerable guidewires to robotic surgery instruments, the company has experience in a variety of specialties that make them well-equipped for any need within the industry. With their commitment to excellence and passion for revolutionizing healthcare technology, NAGL MedTech is a leader in transforming how we approach modern medicine.

07
Xtant Medical
Belgrade, MT
Xtant Medical is a US-based medical device manufacturer that specializes in orthobiologics and spinal fixation systems. Their comprehensive portfolio of innovative products is designed and manufactured in their state-of-the-art tissue processing facility, ensuring the highest quality standards. Customers can take advantage of Xtant Medical's Sales Support team and Product Portal to make orders as well as access resources such as EIFU documents. The company recently completed an acquisition and achieved a revenue growth of 32%, making it a rapidly growing leader in the field. With their commitment to providing superior products backed by excellent customer service, Xtant Medical is sure to be successful in helping people have better lives.

08
Tempo Medical Products
Warren, OH
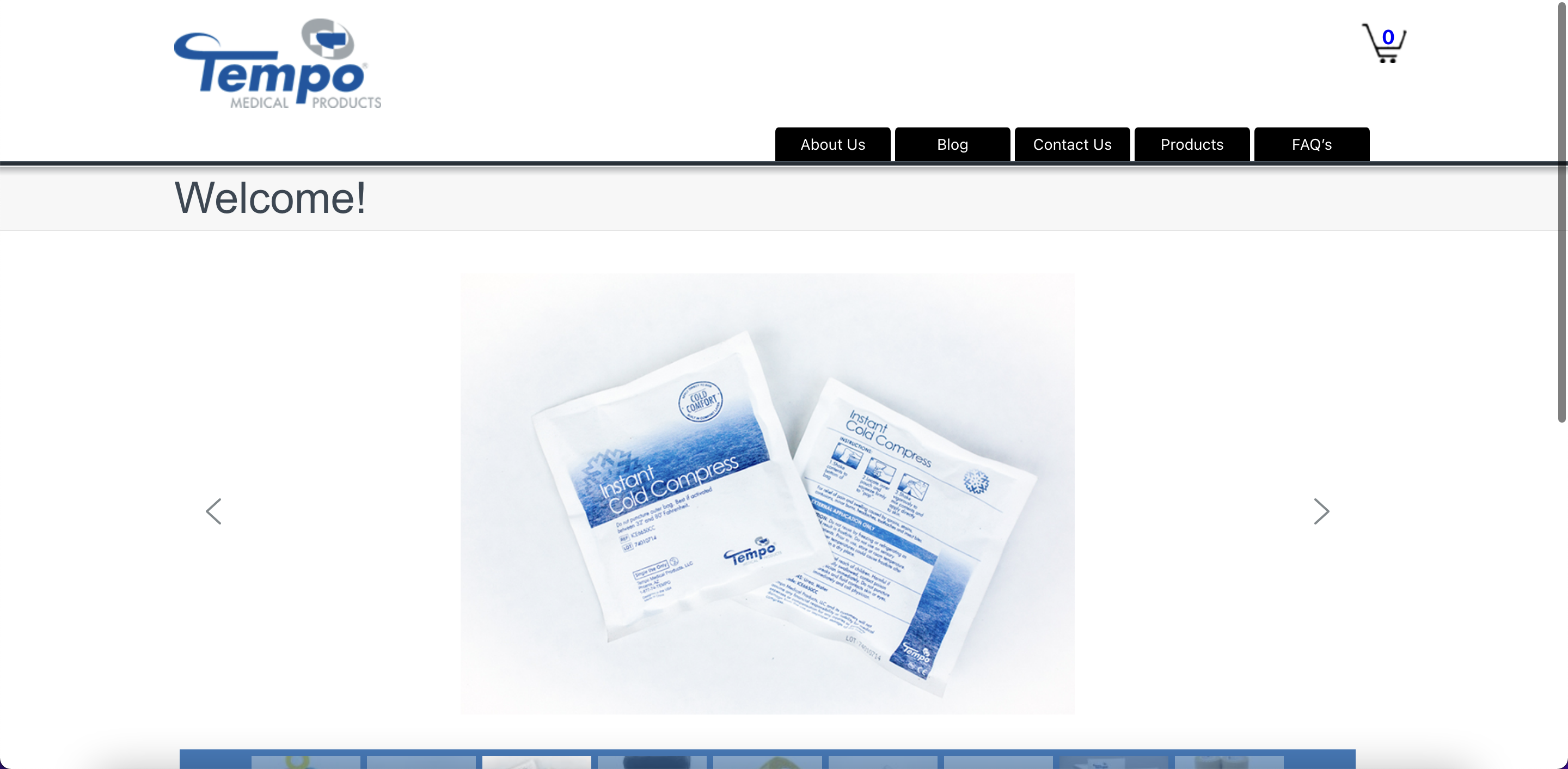
Tempo Medical Products is a US-based medical device manufacturer that provides quality products at competitive prices. They specialize in consumable medical and surgical products designed with patient comfort in mind, and offer customers the unique advantage of sourcing custom products tailored to their specific needs. Their staff is highly dedicated and committed to providing excellent customer service, responsiveness, and support. With a commitment to reducing healthcare costs while enhancing quality of care, Tempo Medical Products is a reliable choice for those seeking top-notch medical device solutions.
09
LKC Technologies
Gaithersburg, MD
Based in the US, LKC Technologies is a leading medical device manufacturer that specializes in electroretinography and visual evoked potentials. They offer an extensive range of products for both humans and animals, such as the RETeval Device, UTAS System, Sensor Strips, RETevet Device, and UTAS BigShot, which are suitable for clinical applications such as retinal ischemic diseases, inherited retinal disorders, and glaucoma. Furthermore, their state-of-the-art technology helps researchers expand their understanding of retinal function with accurate results. Additionally, they provide resources, including research videos, webinars, and testimonials, to help customers get up to speed on their solutions. With a mission focused on helping customers improve patient outcomes through innovative testing devices, LKC Technologies stands out amongst its competition.

10
NeuroOne Medical Technologies Corporation
Eden Prairie, MN
NeuroOne is a medical device manufacturer based in the US, specializing in thin-film electrode technology to advance neuroscience. They have developed Evo® Cortical and Evo® sEEG Electrodes – particularly suited for seizure detection – that are minimally invasive and offer high-definition recordings of neural activity. Their innovative products offer superior scalability, precision, single-tail design, reduced cable management, and decreased immunological response compared to other similar devices on the market. The company is committed to research and development into neurological conditions beyond epilepsy, with support from leading experts in their field. NeuroOne's detailed focus on quality assurance makes it an ideal partner for those looking for reliable medical device solutions in the US.
FAQ
Key questions to consider before hiring a medical device manufacturer
Does the Medical Device Manufacturer have extensive experience in producing medical devices that meet current regulatory standards?
Yes, a seasoned Medical Device Manufacturer typically possesses extensive experience in producing medical devices that comply with current regulatory standards. These standards, often set by governing bodies like the FDA in the U.S. or the European Medicines Agency in Europe, are created to ensure the safety and efficacy of medical devices; hence, compliance is paramount. Notably, the production process for these devices is multifaceted, requiring stringent quality control measures, rigorous testing, and comprehensive documentation. In addition to their technical acumen, these manufacturers often employ regulatory affairs specialists whose role is to stay abreast of the ever-evolving regulatory landscape; this ensures that the devices they produce are always in line with current guidelines. Therefore, when comparing Medical Device Manufacturers, one should carefully consider their track record for regulatory compliance, as this is typically a reliable indicator of their commitment to quality and safety. It's worth noting that while we provide objective reviews of these manufacturers, we do not offer manufacturing services directly.
Does the Medical Device Manufacturer have a track record of delivering quality products on time and at a reasonable cost?
Yes, a track record of delivering quality products on time and at a reasonable cost is a crucial factor when evaluating a Medical Device Manufacturer. Quality, in this context, refers to the production of medical devices that meet rigorous industry standards and regulatory requirements; it also denotes devices that provide reliable, effective outcomes for end-users. Punctuality is another critical component, as healthcare providers often rely on these devices for life-saving treatments and procedures; a delay in delivery can lead to significant negative impacts on patient care. Cost-efficiency, meanwhile, is vital in the healthcare field where budgets are often tight; a manufacturer that can provide quality devices at a reasonable price point is indeed valuable. Hence, when comparing different Medical Device Manufacturers, consider their reputation for quality, timeliness, and cost-effectiveness; these factors are indicative of their ability to meet your needs effectively. It's also advisable to look at customer reviews and ratings, industry reports, and other third-party assessments to get a more comprehensive view of their performance track record. Remember, choosing the right manufacturer can greatly influence the success of healthcare delivery.
Do the Medical Device Manufacturer's production facilities adhere to stringent safety protocols and best practices?
Yes, reputable medical device manufacturers adhere to rigorous safety protocols and best practices in their production facilities. This adherence is not merely a matter of choice, but a regulatory requirement; agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), among others, establish and enforce guidelines that manufacturers must follow. These regulatory bodies insist on compliance with standards like Good Manufacturing Practices (GMP) that govern processes, equipment, and personnel to ensure product safety and efficacy.
Moreover, these companies also frequently subject themselves to third-party audits to validate their adherence to these protocols. Of course, not all manufacturers are created equal; the level of compliance can vary, making it crucial for stakeholders to conduct thorough research or rely on objective, expert reviews. In this complex landscape, a company's commitment to rigorous safety protocols can be a significant differentiator, indicating a dedication to product quality and patient safety.
Understanding this, it becomes clear why regulatory compliance is such a critical factor to consider when comparing medical device manufacturers. So, when delving into the medical device manufacturing space, probing into the safety standards and best practices that manufacturers follow can provide valuable insights to make an informed decision. It's not merely about what devices they produce, but how they produce them; the 'how' speaks volumes about their credibility, reliability, and commitment to patient safety.

01
Katecho
Des Moine, IA
Katecho is an experienced medical device manufacturing company, based in Des Moines, IA. With a commitment to innovation and design, the team at Katecho offers comprehensive services for product design, process design, printing, manufacturing, and logistics. They are certified by ISO 13485:2003 as well as Japanese and Korean government authorities. The company also specializes in hydrogel and pharmaceutical manufacturing solutions. Their passion for building products with purpose has led them to successful products delivered with precision to customers around the world. From product development through the delivery of finished goods, Katecho stands out from other US-based contract medical device manufacturers, providing exceptional value to their clients.

02
Medical Murray
North Barrington, IL
Medical Murray is a contract development and manufacturing organization for minimally invasive medical devices and implants based in the US. They specialize in a range of technologies, from complex catheter delivery systems to steerable and deflectable balloon catheters, and sensor integration with implants. Their experienced team is dedicated to providing quality service, from concept through commercialization. Medical Murray can be trusted for its ISO 10555 Catheter Testing, ISO 25539 Stent Testing, ISO 80369 Luer Testing, and Power Injection testing capabilities, all of which comply with the highest safety standards. With their expertise and collaborative approach, they are sure to provide excellent products that will improve lives around the world.

03
MME Group
St. Paul, MN
MME Group is a full-service injection molder and contract manufacturer based in St. Paul, Minnesota. They specialize in providing comprehensive services to medical device manufacturers, from engineering through assembly. Their vertical integration advantage enables them to provide on-site tooling design and builds, custom plastic and silicone injection molding, secondary operations, high-level assembly, packaging, inventory management, supply chain management, and distribution – all under one roof. In addition to their excellent services, they are ISO 13485:2016 certified, ITAR registered, and have certifications for UL, CE, and TUV, making them the ideal choice for any of your medical device needs. Not only are they trusted by some of the biggest names in manufacturing, but they also offer superior quality control processes, which ensure that each product conforms to industry standards before being shipped out.

04
GCM
Union City, CA
GCM Precision Manufacturing is a trusted medical device manufacturer based in the United States. They specialize in providing high-quality and reliable components for medical technology, surgical robotics, aerospace, and industrial markets. Their services include engineering, supply chain management, precision machining, robotic welding and joining technologies, automated sheet metal fabrication, precision assembly, and more. With over 40 years of experience in key end markets such as MedTech, Aerospace, and Industrial applications, they continue to invest in their talent and technology infrastructure to provide industry-leading solutions. Their commitment to quality has been unwavering, as seen through their continual improvement of Quality Management Systems, designed to exceed customer expectations.

05
ViewRay
Oakwood Village, OH
ViewRay is a medical device manufacturer in the US, specializing in MRI-guided radiation therapy. Their groundbreaking work has enabled them to treat over 31,000 patients worldwide with their advanced A3i system. Patients benefit from improved imaging capabilities and increased accuracy in radiation delivery for various cancers such as pancreas, prostate, lung, liver, and breast cancer. Clinicians can also take advantage of the company's extensive clinical trials and Investigator-Initiated Research programs to further explore the possibilities offered by ViewRay's technology. The leadership team at ViewRay is dedicated to providing innovative solutions that make a real difference in cancer care.

06
NAGL Medtech
Miramar, FL
NAGL MedTech is a US-based medical device manufacturer specializing in transforming healthcare through cutting-edge technologies. The company provides comprehensive services for development, manufacturing, and consulting, offering clients the full spectrum of solutions necessary to create innovative medical devices. NAGL MedTech's team of experts is knowledgeable in every aspect of the industry and collaborate closely with their clients to provide strategic solutions tailored to their challenges and objectives. From neurovascular steerable guidewires to robotic surgery instruments, the company has experience in a variety of specialties that make them well-equipped for any need within the industry. With their commitment to excellence and passion for revolutionizing healthcare technology, NAGL MedTech is a leader in transforming how we approach modern medicine.

07
Xtant Medical
Belgrade, MT
Xtant Medical is a US-based medical device manufacturer that specializes in orthobiologics and spinal fixation systems. Their comprehensive portfolio of innovative products is designed and manufactured in their state-of-the-art tissue processing facility, ensuring the highest quality standards. Customers can take advantage of Xtant Medical's Sales Support team and Product Portal to make orders as well as access resources such as EIFU documents. The company recently completed an acquisition and achieved a revenue growth of 32%, making it a rapidly growing leader in the field. With their commitment to providing superior products backed by excellent customer service, Xtant Medical is sure to be successful in helping people have better lives.

08
Tempo Medical Products
Warren, OH
Tempo Medical Products is a US-based medical device manufacturer that provides quality products at competitive prices. They specialize in consumable medical and surgical products designed with patient comfort in mind, and offer customers the unique advantage of sourcing custom products tailored to their specific needs. Their staff is highly dedicated and committed to providing excellent customer service, responsiveness, and support. With a commitment to reducing healthcare costs while enhancing quality of care, Tempo Medical Products is a reliable choice for those seeking top-notch medical device solutions.
09
LKC Technologies
Gaithersburg, MD
Based in the US, LKC Technologies is a leading medical device manufacturer that specializes in electroretinography and visual evoked potentials. They offer an extensive range of products for both humans and animals, such as the RETeval Device, UTAS System, Sensor Strips, RETevet Device, and UTAS BigShot, which are suitable for clinical applications such as retinal ischemic diseases, inherited retinal disorders, and glaucoma. Furthermore, their state-of-the-art technology helps researchers expand their understanding of retinal function with accurate results. Additionally, they provide resources, including research videos, webinars, and testimonials, to help customers get up to speed on their solutions. With a mission focused on helping customers improve patient outcomes through innovative testing devices, LKC Technologies stands out amongst its competition.

10
NeuroOne Medical Technologies Corporation
Eden Prairie, MN
NeuroOne is a medical device manufacturer based in the US, specializing in thin-film electrode technology to advance neuroscience. They have developed Evo® Cortical and Evo® sEEG Electrodes – particularly suited for seizure detection – that are minimally invasive and offer high-definition recordings of neural activity. Their innovative products offer superior scalability, precision, single-tail design, reduced cable management, and decreased immunological response compared to other similar devices on the market. The company is committed to research and development into neurological conditions beyond epilepsy, with support from leading experts in their field. NeuroOne's detailed focus on quality assurance makes it an ideal partner for those looking for reliable medical device solutions in the US.
Frequently Asked Questions
Key questions to consider before hiring a medical device manufacturer
Does the Medical Device Manufacturer have extensive experience in producing medical devices that meet current regulatory standards?
Yes, a seasoned Medical Device Manufacturer typically possesses extensive experience in producing medical devices that comply with current regulatory standards. These standards, often set by governing bodies like the FDA in the U.S. or the European Medicines Agency in Europe, are created to ensure the safety and efficacy of medical devices; hence, compliance is paramount. Notably, the production process for these devices is multifaceted, requiring stringent quality control measures, rigorous testing, and comprehensive documentation. In addition to their technical acumen, these manufacturers often employ regulatory affairs specialists whose role is to stay abreast of the ever-evolving regulatory landscape; this ensures that the devices they produce are always in line with current guidelines. Therefore, when comparing Medical Device Manufacturers, one should carefully consider their track record for regulatory compliance, as this is typically a reliable indicator of their commitment to quality and safety. It's worth noting that while we provide objective reviews of these manufacturers, we do not offer manufacturing services directly.
Does the Medical Device Manufacturer have a track record of delivering quality products on time and at a reasonable cost?
Yes, a track record of delivering quality products on time and at a reasonable cost is a crucial factor when evaluating a Medical Device Manufacturer. Quality, in this context, refers to the production of medical devices that meet rigorous industry standards and regulatory requirements; it also denotes devices that provide reliable, effective outcomes for end-users. Punctuality is another critical component, as healthcare providers often rely on these devices for life-saving treatments and procedures; a delay in delivery can lead to significant negative impacts on patient care. Cost-efficiency, meanwhile, is vital in the healthcare field where budgets are often tight; a manufacturer that can provide quality devices at a reasonable price point is indeed valuable. Hence, when comparing different Medical Device Manufacturers, consider their reputation for quality, timeliness, and cost-effectiveness; these factors are indicative of their ability to meet your needs effectively. It's also advisable to look at customer reviews and ratings, industry reports, and other third-party assessments to get a more comprehensive view of their performance track record. Remember, choosing the right manufacturer can greatly influence the success of healthcare delivery.
Do the Medical Device Manufacturer's production facilities adhere to stringent safety protocols and best practices?
Yes, reputable medical device manufacturers adhere to rigorous safety protocols and best practices in their production facilities. This adherence is not merely a matter of choice, but a regulatory requirement; agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), among others, establish and enforce guidelines that manufacturers must follow. These regulatory bodies insist on compliance with standards like Good Manufacturing Practices (GMP) that govern processes, equipment, and personnel to ensure product safety and efficacy.
Moreover, these companies also frequently subject themselves to third-party audits to validate their adherence to these protocols. Of course, not all manufacturers are created equal; the level of compliance can vary, making it crucial for stakeholders to conduct thorough research or rely on objective, expert reviews. In this complex landscape, a company's commitment to rigorous safety protocols can be a significant differentiator, indicating a dedication to product quality and patient safety.
Understanding this, it becomes clear why regulatory compliance is such a critical factor to consider when comparing medical device manufacturers. So, when delving into the medical device manufacturing space, probing into the safety standards and best practices that manufacturers follow can provide valuable insights to make an informed decision. It's not merely about what devices they produce, but how they produce them; the 'how' speaks volumes about their credibility, reliability, and commitment to patient safety.

